Regenflex / Regenflex Starter / Regenflex BIO-PLUS

REGENFLEX® is a medical device based on a highly-purified Hyaluronic Acid, with molecular weight between 800-1.200 kDalton.
Sodium hyaluronate is a fundamental component of the synovial fluid, as it is responsible of the visco-elastic properties of the latter.
The sodium hyaluronate contained in REGENFLEX® is obtained by means of fermentation, avoiding any kind of chemical modification treatment, in order to guarantee an absolutely pure product, free of remains alien to the physiological context of implementation.
The packages include one single-use syringe, containing 2ml of sterile apyrogen solution.



Cross-linked & linear intercalated Hyaluronic Acid
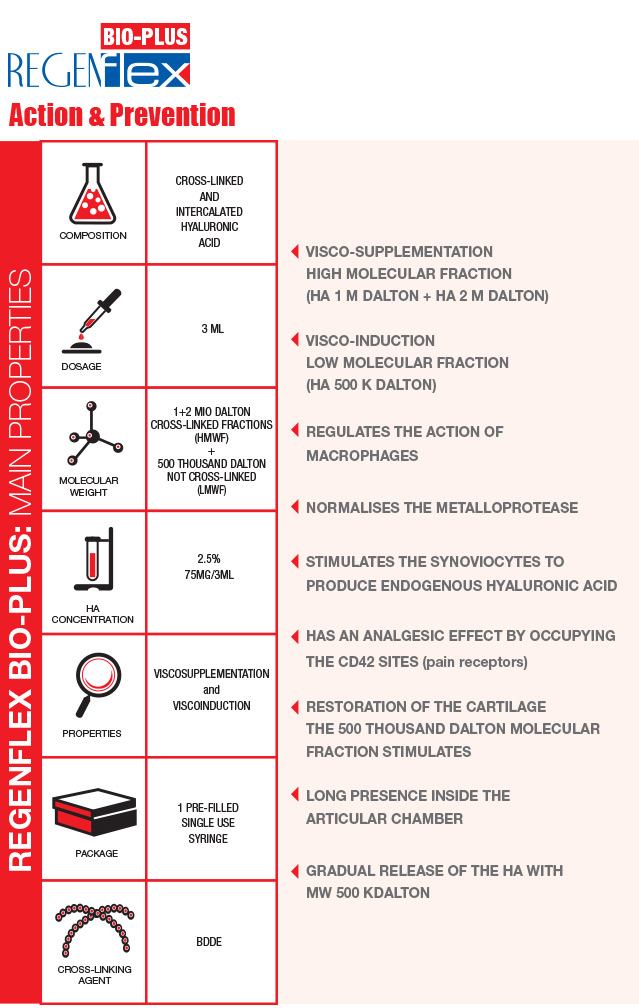
Regenflex BIO-PLUS is a transparent gel based on cross-linked and linear intercalated Hyaluronic acid, created with an advanced and patented technological process.
The aim of this intra-articular injectable is to prevent or to reduce pain, restoring the normal damping and lubricating function of the joint.
Not only will the joint function improve, but the patient will also experience an improvement in their quality of life.
The matter of weight: 2 molecular fractions
The action of the Hyaluronic acid depends on its molecular weight. The weight, in fact, influences the quantity of exogenous hyaluronic acid that can penetrate the synovial membrane: such quantity is proportionally inverse to the molecular weight itself. The close combination of the cross-linked and the intercalated linear Hyaluronic acid, makes it possible to inject two molecular fractions:

Regenflex BIO-PLUS & Biorivolumetria
BIORIVOLUMETRIA is an innovative concept in the field of HYALURONIC ACID-based injectiable medical devices.
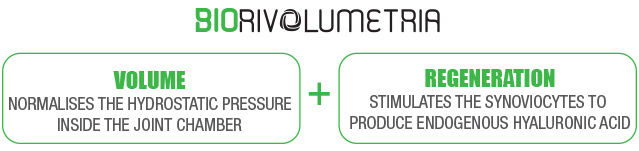
The same product makes it possible to simultaneously: restore the volume of the joint chamber by injecting CROSS-LINKED HYALURONIC ACID, and to stimulate receptors of the synoviocytes and chondrocytes thanks to the gradually released linear fraction of the INTERCALATED HA.

Manufacturing technology and functioning
The manufacturing technology used to produce Regenflex BIO-PLUS is the one used for BIORIVOLUMETRIA. The Biorivolumetria products are MONO-PHASE GELS obtained through a long-lasting cross-linking process at low temperature, the “gentle cross-linking”. The slow mixing allows for the cross-linking agent (BDDE) to widespread in a better and more uniform way. The amount of BDDE used is 30 per cent lower than in the majority of other products with equal degree of viscosity currently on the market. For the manufacturing of Regenflex BIO-PLUS three molecular weights of Hyaluronic Acid are used: 1 M, 2 M and 500 THOUSAND Dalton. The latter is linear and intercalated and represent 10 per cent of the total Hyaluronic Acid. Differently long chains of HA help to better calibrate the product’s viscosity. The linear Hyaluronic Acid is released gradually in time, performing a prolonged biological action (visco-induction).
Intercalation process






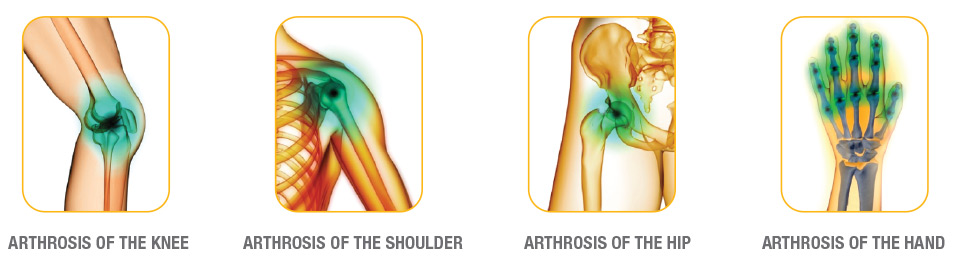
 In the case of chondral diseases, the bones, which in normal conditions should slide against each other without friction because of their smooth and flexible tissue, are instead impaired in the movements.
This is due to the loss of rheologic synovial properties, that causes a notable reduction of the synovial fluid’s visco-elasticity, leading to:
In the case of chondral diseases, the bones, which in normal conditions should slide against each other without friction because of their smooth and flexible tissue, are instead impaired in the movements.
This is due to the loss of rheologic synovial properties, that causes a notable reduction of the synovial fluid’s visco-elasticity, leading to: